Our CRAs team is located in 2 regions, in the North and South of France :
- North : in Normandy, near PARIS
- South : in the South-West, near TOULOUSE
We operate throughout France and mainly in the following cities :
Our CRAs team is located in 2 regions, in the North and South of France :
We operate throughout France and mainly in the following cities :
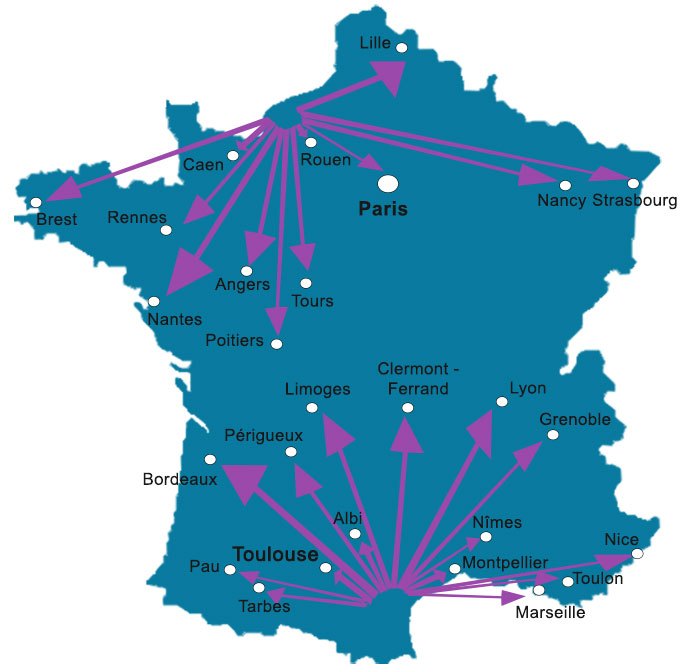
Travel throughout Metropolitan France and the French overseas departments and territories
We also operate in Europe and abroad in countries such as Belgium, England, Switzerland, Spain, Germany and Australia.